Learning about Atomic Spectra & Neon Signs
October 15, 2024
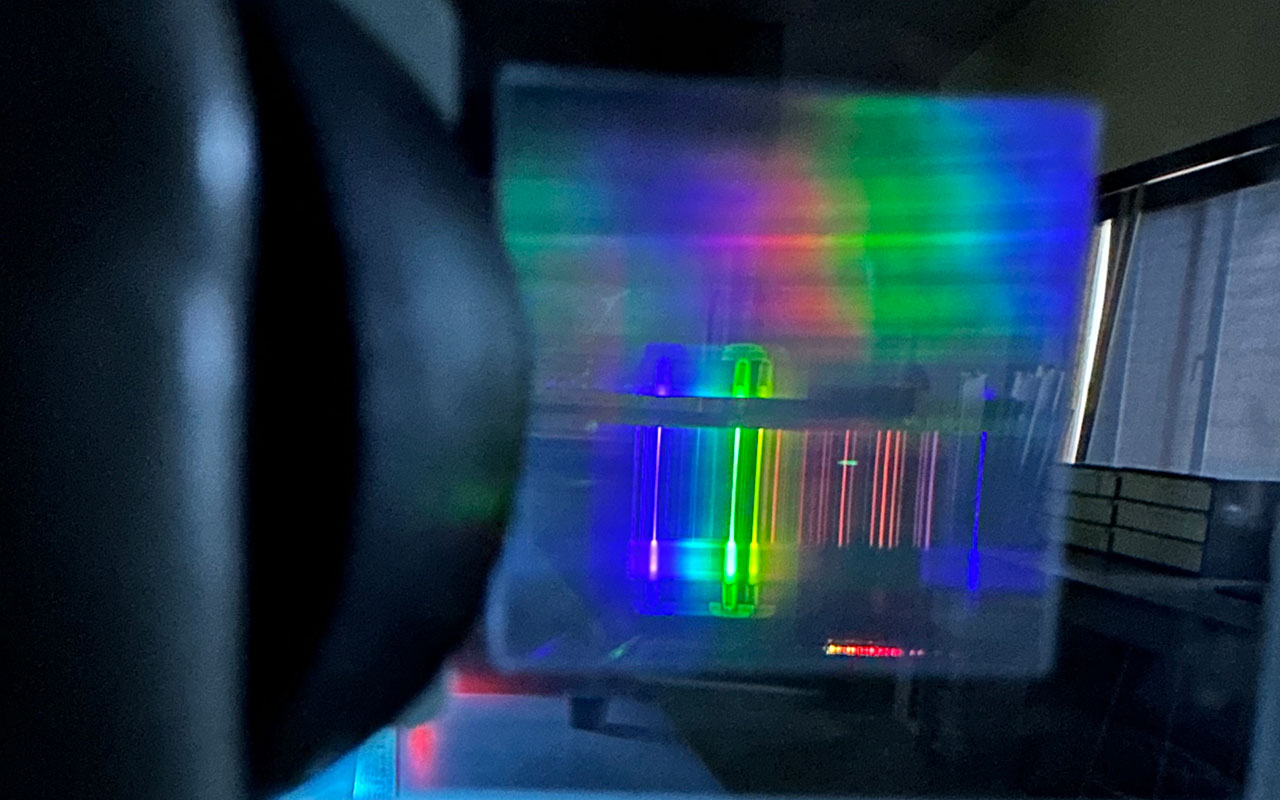
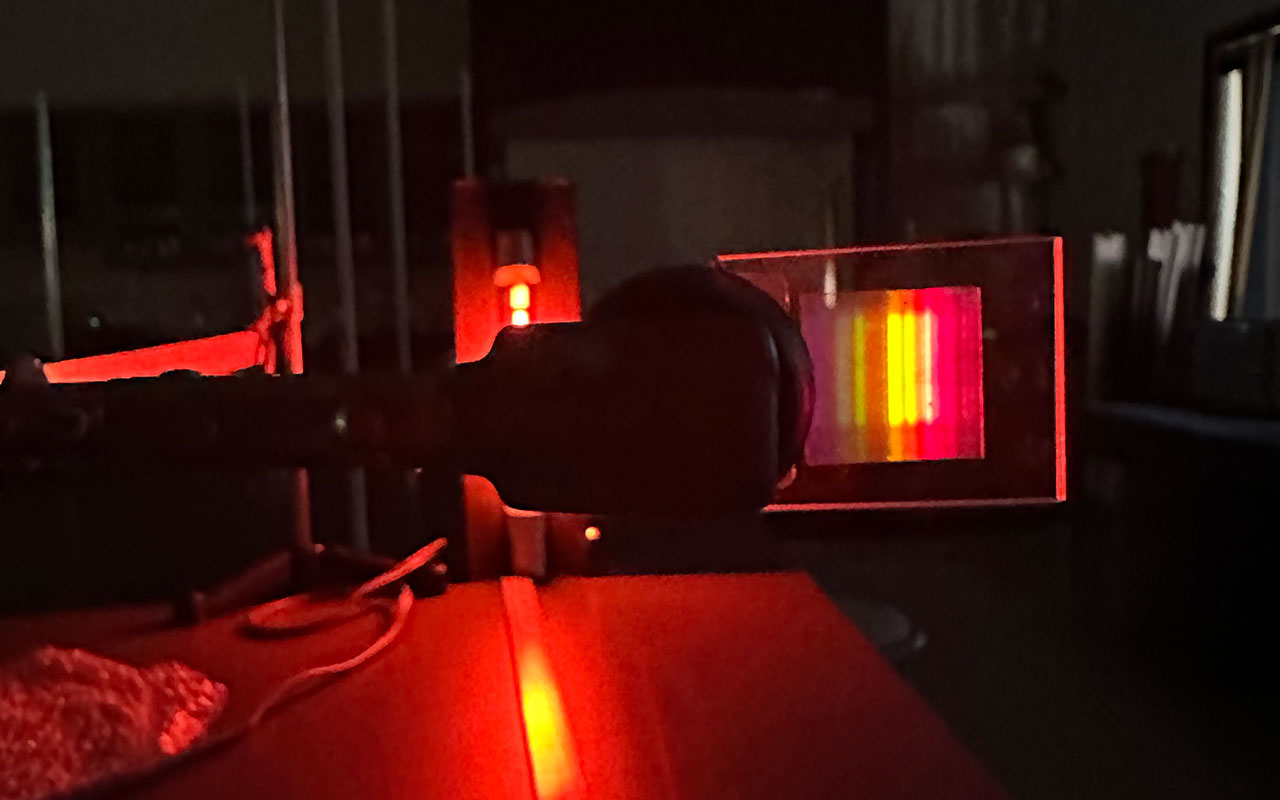
Students in Carl Franz's Chemistry Lab explored Atomic Spectra emissions of different elements by passing a strong current through tubes of glass. This is how common neon signs get their beautiful glow.

The secret lies in atomic emission spectra. When a strong current passes through gas-filled tubes, the atoms in the gas get excited, and their electrons jump to higher energy levels. As the electrons return to their original state, they emit light specific to the element, creating distinct colors. Neon produces its famous red-orange glow, while other gases like argon or krypton create blues and greens. This process mirrors our work studying the light patterns of different elements, a fundamental technique in both science and industry.
Sources: Nicole Kandi - stock.adobe.com, Physics LibreTexts, Neon Signs - Science of Lighting